Give the Major Force Between Ethanol and Water
You need to convert 100 g of H2O to moles because ΔHfus is in moles. Choose the bond below that is most polar.

Gluten Production Process Diagram Floor Plans Process
Give the major force between ethanol and waterA.

. A solution will form between two substances. D A solution will form between two substances only if the solvent-solvent interactions are weak enough to overcome the solute-solvent interactions. A dipole-dipole B dispersion C hydrogen bonding D ion-ion E ion-dipole C D Ans.
Place the following in. 2 Give the major force between ethanol and water. Correct answer to the question Give the major force between ethanol and water.
Give the major force between ethanol and water. Sign up for free. Give the major force between ethanol and rubbing alcohol.
Asked Aug 7 2019 in Chemistry by Hardwell. Give the approximate bond angle for a molecule with an octahedral shape. 1 Choose the statement below that is TRUE.
There is no chemical reaction between ethanol and water. Royal Society of Chemistry. If the solute-solvent interactions are of comparable.
Calculate the mass of nitrogen dissolved at room temperature in an 840 L home aquarium. Dispersion Ion-Dipole Ion-Ion Dipole-Dipole Hydrogen Bonding. The Henrys law constant for oxygen in water at 25C is 13 10-3 Matm.
E ion-dipole 3 Give the major force in seawater. However there is an interaction. C 2 S B NBr 3 C F 2 6.
Alcohol and water are both excellent solvents using hydrogen bonds to dissolve the solute. Hydrogen bonding oxygen bonding dipole-dipole ion-ion ionic forces. Give the major force between ethanol and water.
105 90 Place the following in order of INCREASING dipole moment. E None of the above are true. In a solution of water and ethanol hydrogen bonding is the strongest intermolecular force between molecules.
A double covalent bond contains __________ of electrons. Give the major force between ethanol and water. Melt solid ice to liquid water at 0ºC.
Give the major force between ethanol and water. 84 379 ratings Sign up for free to keep watching this solution. 7 Give the major force between acetone and chloroform draw the molecules.
Hydrogen bonding If two substances are soluble in all proportions then they are ________ in each other. Hydrogen bond are intermolecular force that are weaker than covalent bond but holds atoms together in a molecules. The major force between ethanol and rubbing alcohol is hydrogen bond.
A 0 pairs B 1 pair C 2 pairs D 3 pairs E 4 pairs Ans. Give the major force between ethanol and rubbing alcohol. A A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions.
Give the major force between ethanol and water. Hydrogen bonding is a type of interactions between molecules that occurs when a partially negative atom such as oxygen end of one of the molecules is attracted to a partially positive hydrogen end of another molecule. Give the major force between ethanol and rubbing alcohol.
A solution of water and ethanol contains the dipole-dipole forces and hydrogen bonds as the intermolecular forces between molecules. Hydrogen bonding occurs when the partially negative oxygen end of one of the molecules is attracted to the partially positive hydrogen end of another molecule. Ion-ion dipole-dipole ion-dipole dispersion hydrogen bonding.
At 35 C the vapor pressure of chloroform is 0359 atm and that of acetone is 0453 atm. Answer 1 of 28. Give the major force between acetone and chloroform.
When heating within a phase use q m Cs ΔT. A dipole-dipole B dispersion C hydrogen bonding D ion-ion E ion-dipole. A 0454 B 0220 C 0818 D 0122 E 0153 m m m m m.
2 Give the major force between ethanol and water. When performing a phase change between solid and liquid use qnΔHfus Note. Q 100g 209 JgK 50K 1045 J.
Ion-ion dipole-dipole ion-dipole dispersion hydrogen bonding. 8 Determine the molality of an aqueous solution prepared by dissolving 0112 moles of LiCl in 137 moles of H 2 O. If you add water to alcohol the mixture would be a.
Determine the partial pressure of oxygen necessary to form an aqueous solution that is 65 10-4 M O2 at 25C. Give the major force between ethanol and water. For an hydrogen bond to be formed a molecule must contain an hydrogen atom that will be bonded to one of the most electronegative element.
Assuming ideal behavior what is the vapor pressure of chloroform above a solution containing 100 mole of chloroform and 400 moles of. Hydrogen bonding oxygen bonding dipole-dipole ion-ion ionic forces.

Will Ethanol Float Or Sink In Water Quora

Will Ethanol Float Or Sink In Water Quora

New Yorker Cartoon Artist Nature Journal Awesome Cartoon Artist Nature Journal New Yorker Cartoons
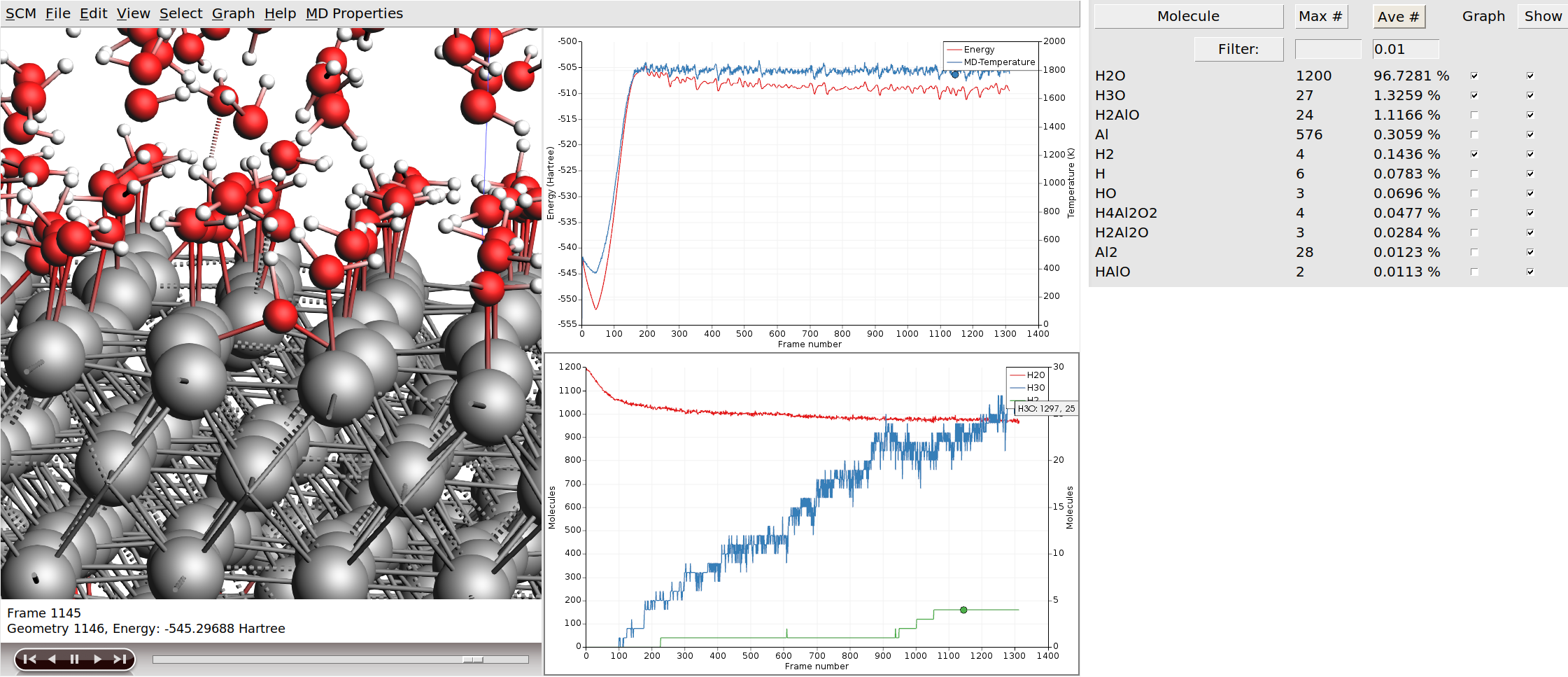
Solid Liquid Interface Tutorials 2022 1 Documentation

Discovering The Link Between Nutrition And Skin Aging Disease Disorders Cardiovascular

Characteristics Of Fluids Fluid Mechanical Engineering Characteristics

Woohoo All Natural Deodorant Paste Wild Super Strong Eco 60g 2 12oz Toxin Free Woohoo Deodorant All Natural Deodorant Natural Deodorant Deodorant

Non Solid Fuel Gaining Popularity Around The World Financial Inclusion Financial Country

12 6 Types Of Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts
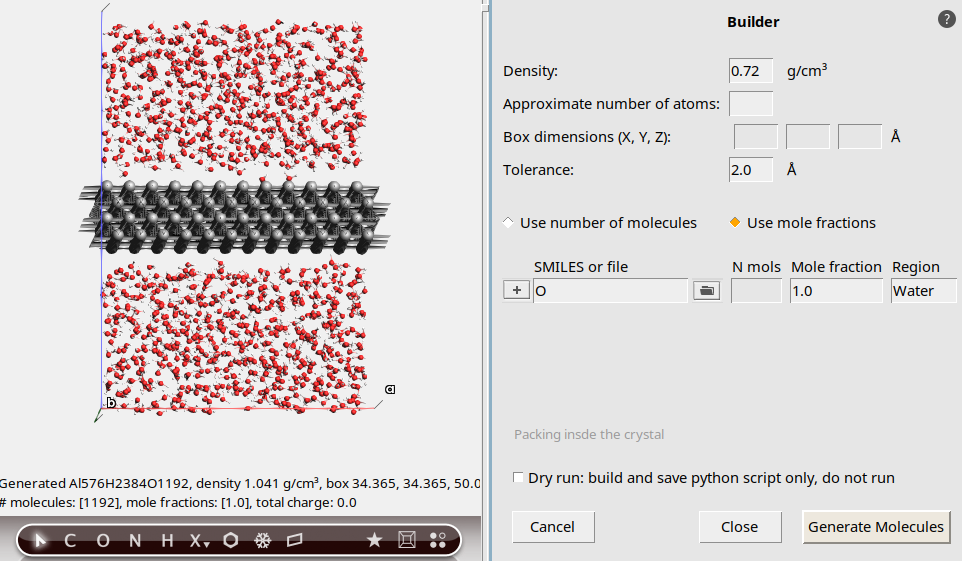
Solid Liquid Interface Tutorials 2022 1 Documentation

Column Design Reinforcement In Column Steel Column Column Design Steel Columns Concrete Column

Chemistry The Central Science Chapter 11 Section 2 Chemistry Lessons Intermolecular Force Teaching Chemistry
A Surface Tension Of The Ethanol Water Mixture As A Function Of Download Scientific Diagram

What Is The Strongest Intermolecular Force Between Water And Ethanol
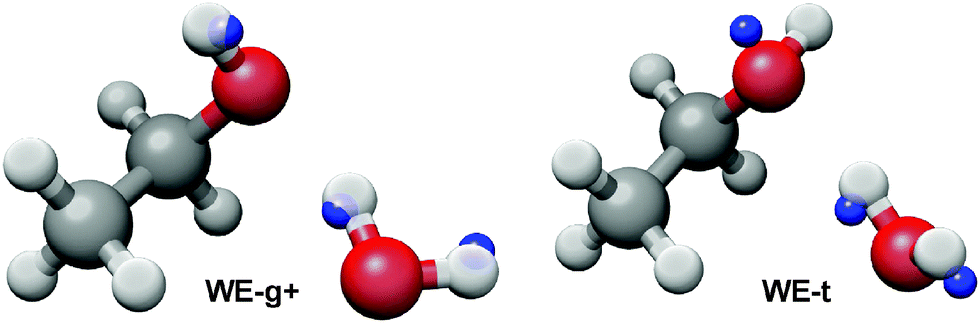
Hydrogen Bonding In The Ethanol Water Dimer Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C5cp03589a

Copper Mining And Processing Copper Mining Coal
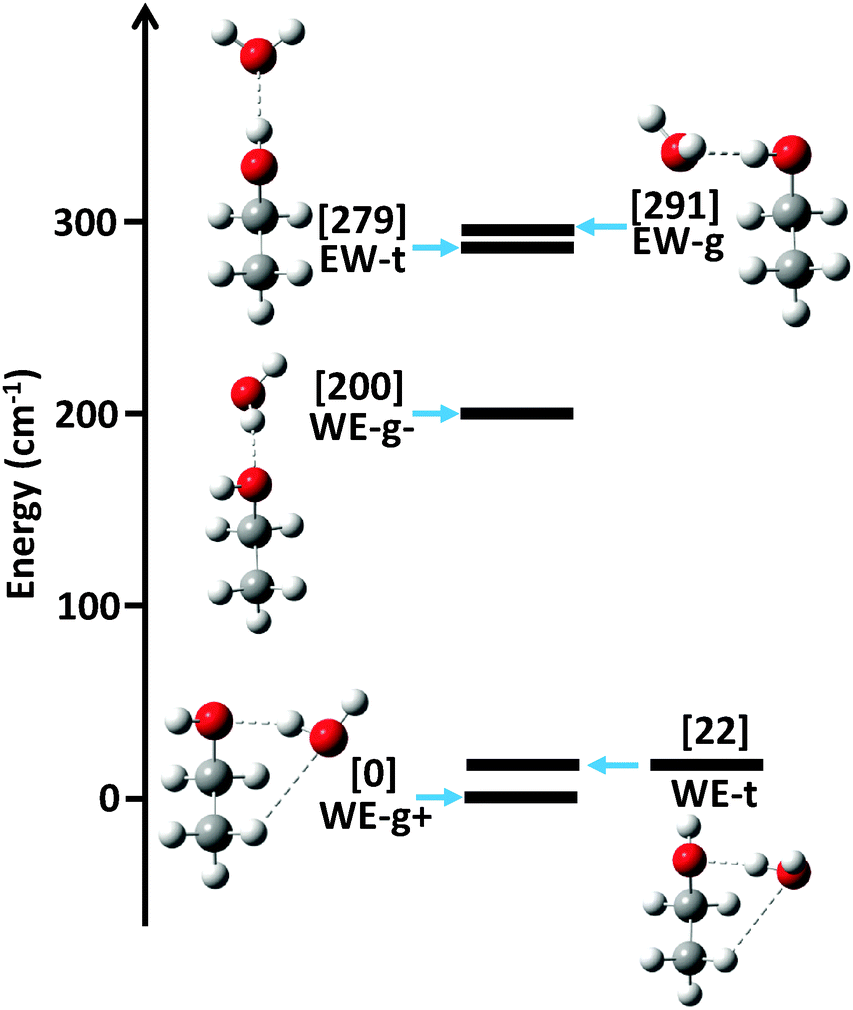
Hydrogen Bonding In The Ethanol Water Dimer Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C5cp03589a

Comments
Post a Comment